Moles
In Topic B.1, you learned about phases, phase changes, and heat transfer of substances. Whilst it is easy to perform experiments measuring these phenomena in solids and liquids due to their finite volume, it completely changes in gases.
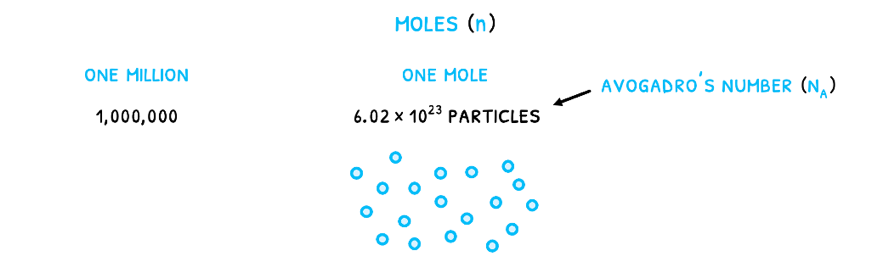
The change is that gases cannot easily be measured by their mass or volume. As a result, they are measured in moles (n). This is a measure of the number of particles given by Avogadro’s number (NA) equal to 6.022 x 1023 particles/mole. It relates to the number of particles that would make Carbon-12 exactly 12 grams as the standard element. Therefore, just like one million is 1,000,000, one mole is 6.022 x 1023 particles. For any number of particles (N), to calculate the number of moles, use the formula:
n=NAN
Note that this describes the number of particles so 1 mole of any diatomic molecule, such as H2, has 1.2044 x 1024 atoms because every molecule (and therefore particle) has two component atoms.
The mass of an atom can be expressed as molar mass (M), the mass of one mole of a substance in gmol-1. Calculating molar mass is possible with the formula:
M=nm
Pressure
Once you know how many moles of gas you have, you can then start to analyze its behavior when changing several variables, including pressure, temperature, and volume.
Whilst we know now what temperature is, and the concept of volume is straightforward, we don't know much about what pressure is yet.
In IB physics, you are supposed to understand that the amount of pressure a gas inside a container experiences is related to the force of the particle collisions with the container walls and the number of collisions per area. To easily digest this concept, think of getting poked and bumped.
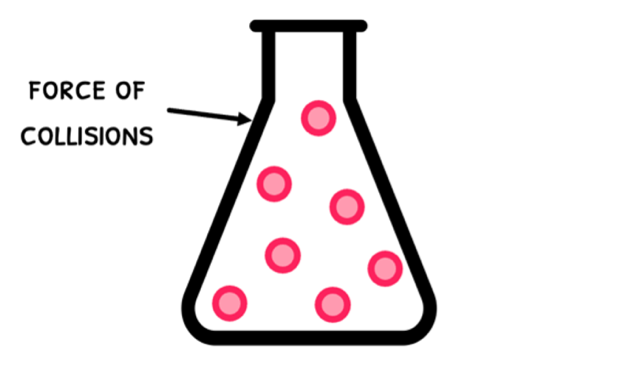
- If you get poked softly, you don't feel much pressure. If you get poked hard, suddenly you feel the pressure. The same occurs with particles - collisions with little force don't exert much pressure whereas collisions with high force do exert considerable pressure.
- However, now compare poking and bumping with the same force. A soft bump would result in less pressure than a soft poke because it is spread out over a larger area. The same occurs with particles - less collisions over the same area result in a lower pressure than many collisions over the same area.
Therefore, pressure is the force per unit area of the container. The formula for this is:
P=AF
However, whenever a force is exerted on the container to cause this pressure, the particle transfers energy to the container. This change in energy is related to a change in speed and thus momentum of the particle. As a result, pressure can also be defined in terms of the change of momentum. The formula for this is:
P=31ρv2

