Phases of matter
In Topic A.3, considerable time was taken in expanding on the types of energy you will encounter during IB physics. However, when considering the behavior of energy and how it transfers between objects, you need to understand how the particles within objects behave to store and release energy.
To begin, remember that each object can present itself as a state of matter (solid, liquid or gas) but can switch between them. The kinetic theory governs the properties of each phase: solids, liquids, and gases.

- Solids have a fixed volume and shape because their particles vibrates around a fixed position held there by strong intermolecular forces. This is typically the densest phase.
- Liquids have a fixed volume and fill the shape of their container, because their particles slide over one another due to weaker intermolecular force. This phase typically has an intermediate density.
- Gases do not have a fixed volume and fill the shape of thier container, because their particles move randomly in space due to their very weak intermolecular forces. This phase typically has the lowest density.
Note that density is the ratio of mass to volume. The formula for this is:
ρ=Vm
Temperature
Now it is common sense that what separates solids from liquids from gases is temperature. Relative to one another, you might assume that a solid is cold, a liquid is warm, and a gas is hot. What is less obvious is what temperature means.
An object's temperature (T) is the average kinetic energy of all its particles. So, the faster particles in an object move, the warmer the object is, and the colder particles in an object, the colder the object is.
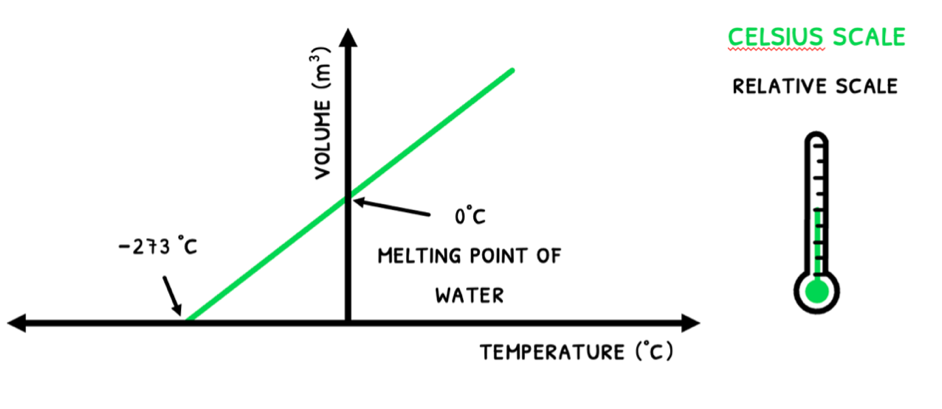
In day-to-day situations, temperature is commonly measured in Celsius (°C). This is a scale based off water, where 0°C is the melting/freezing point of water and 100°C is the boiling/condensing point of water. However, this is not a very scientific way to measure kinetic energy.
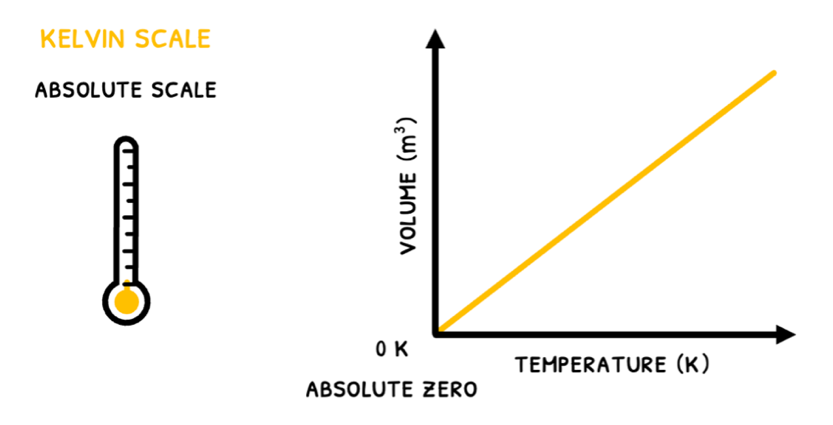
As a result, the Kelvin scale was created. This measures temperature in Kelvin (K), based off 0 K being absolute zero, the coldest possible temperature. At this point, the particle have zero kinetic energy.


