Fission
Now that you have learned the basics of atoms and radiation, you need to understand how energy is produced in nuclear reactors. There are two types of nuclear reactions:
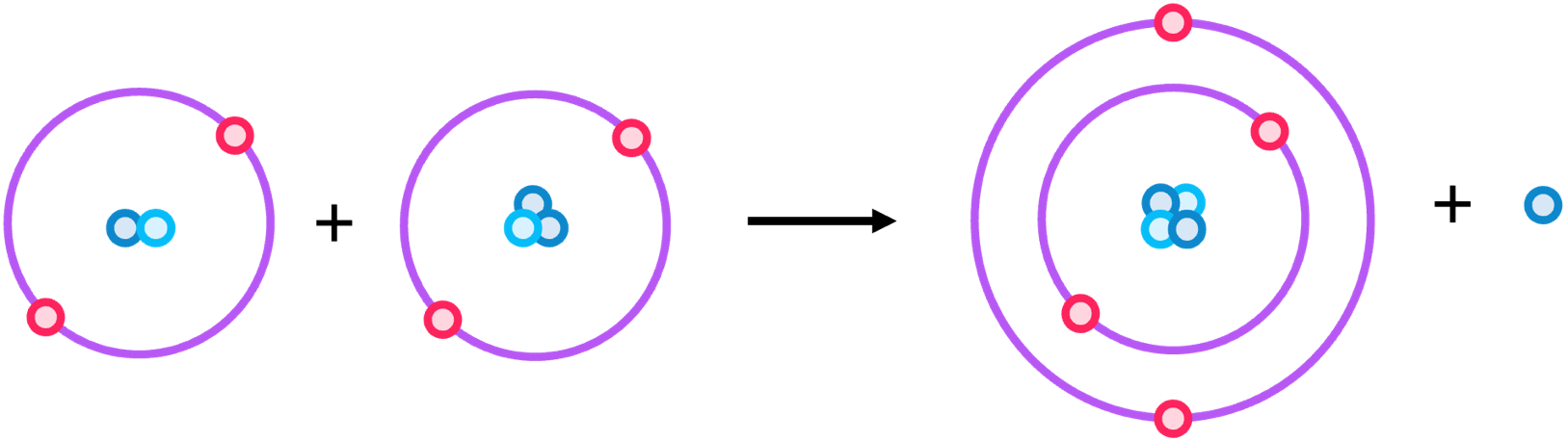
Fusion - the joining of two nuclei to form a larger nucleus, releasing lots of energy.
An example is the fusion of H-2 and H-3 to form He-4 and one neutron. This is the process that occurs in the sun.
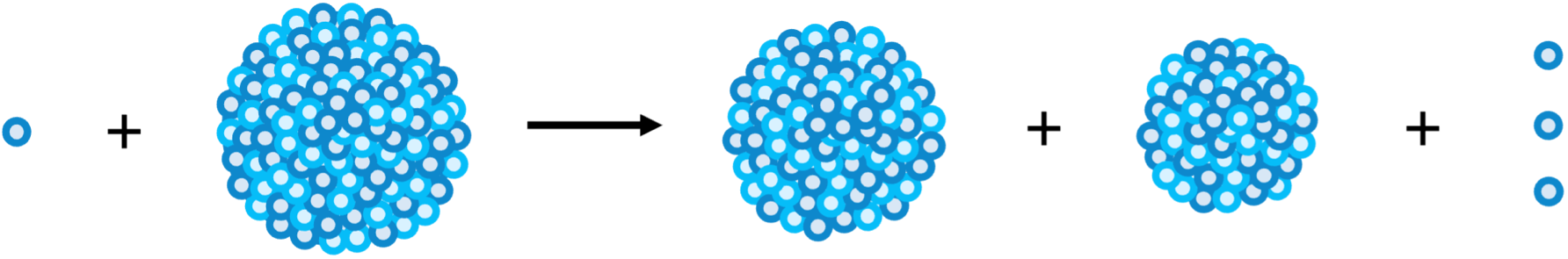
Fission - the splitting of a large nucleus into two smaller nuclei, releasing less energy, although still a large amount.
An example is the fission of a U-235 nucleus by a neutron into Ba-141, Kr-92 and 3 neutrons. This is the process that occurs in nuclear reactors.
Whilst fusion has a higher energy output, it currently requires a higher energy input to initiate and is thus not industrially viable for now. Thus, fission is the current reaction used in nuclear reactors.
Chain reaction
In a reactor, fission can be initiated one of two ways:
- Spontaneously - radioactive elements like Uranium are already unstable and will undergo radioactive decay by themselves without any external energy input. However, this occurs at low baseline levels and yields little energy.
- Neutron-induced - a neutron is fired at Uranium nucleus, colliding and making it more unstable. This instantly induces fission .
Following the initiation of fission reactions, remember that a Uranium nucleus will release three neutrons. At sufficient speeds, these can set off the fission of three other nuclei, with each setting off another three etc, called a chain reaction.
Due to the exponential growth of fission in a chain reaction, harnessing fission power is incredibly dangerous and needs to be well-monitored and controlled.

